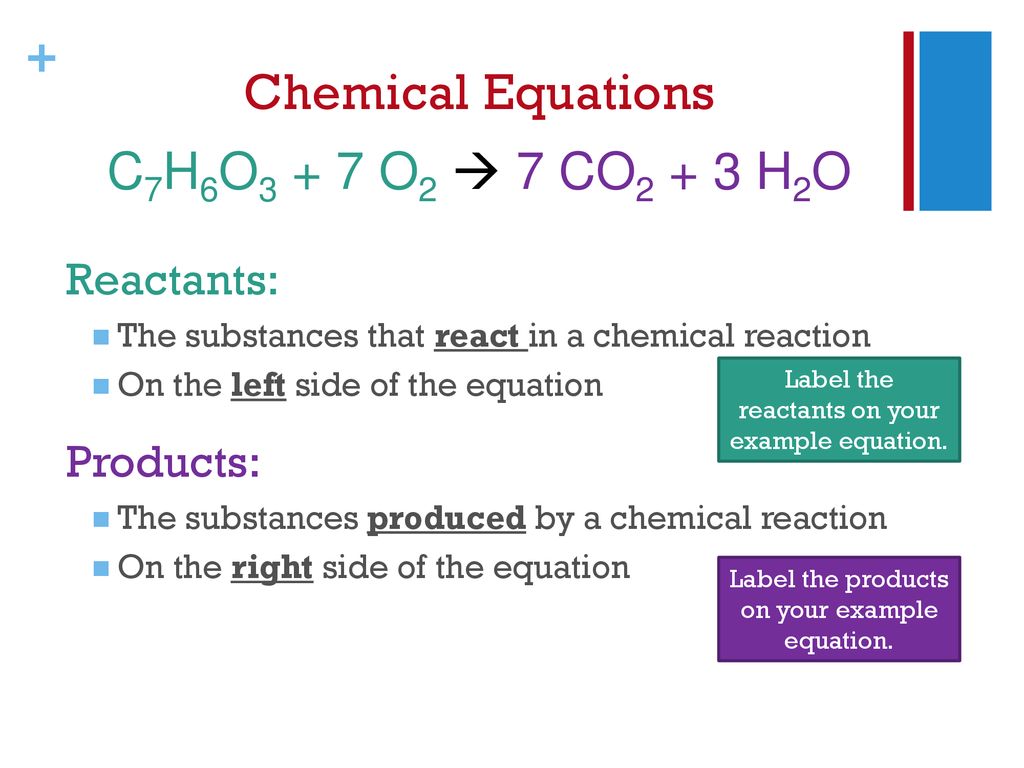
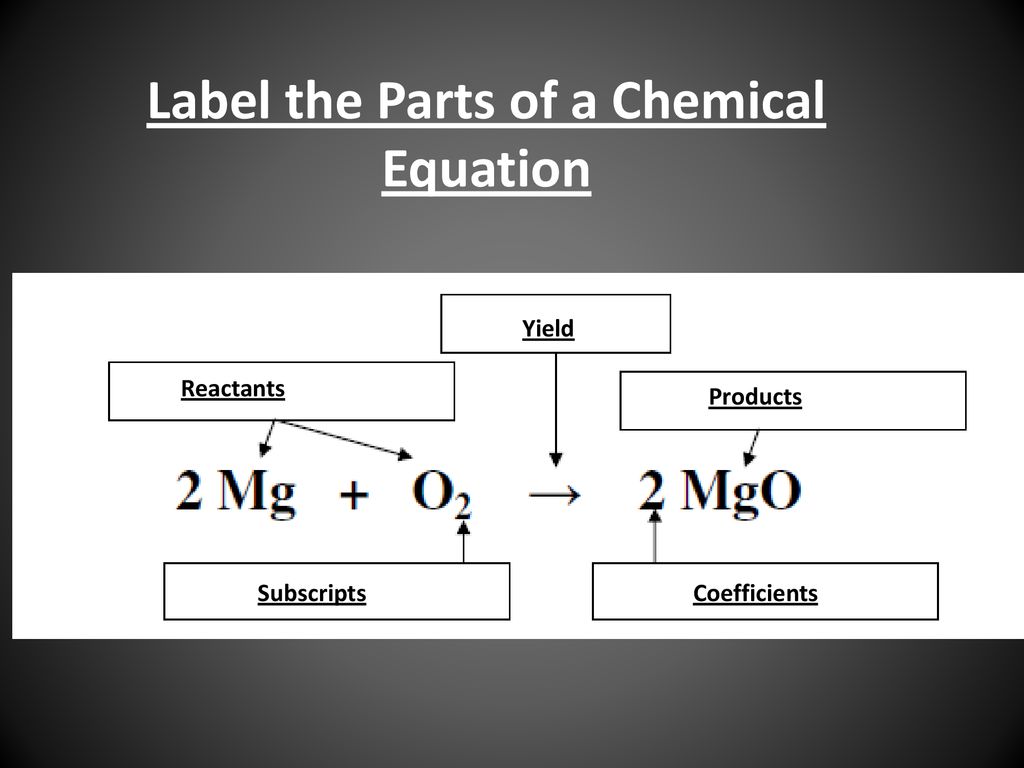
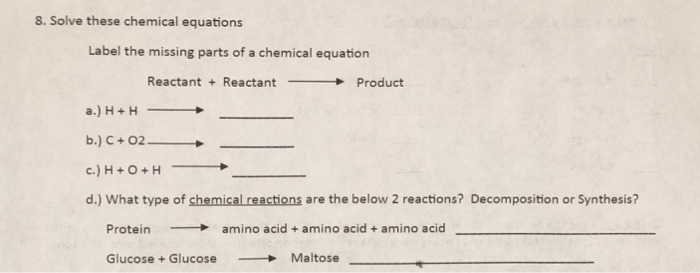
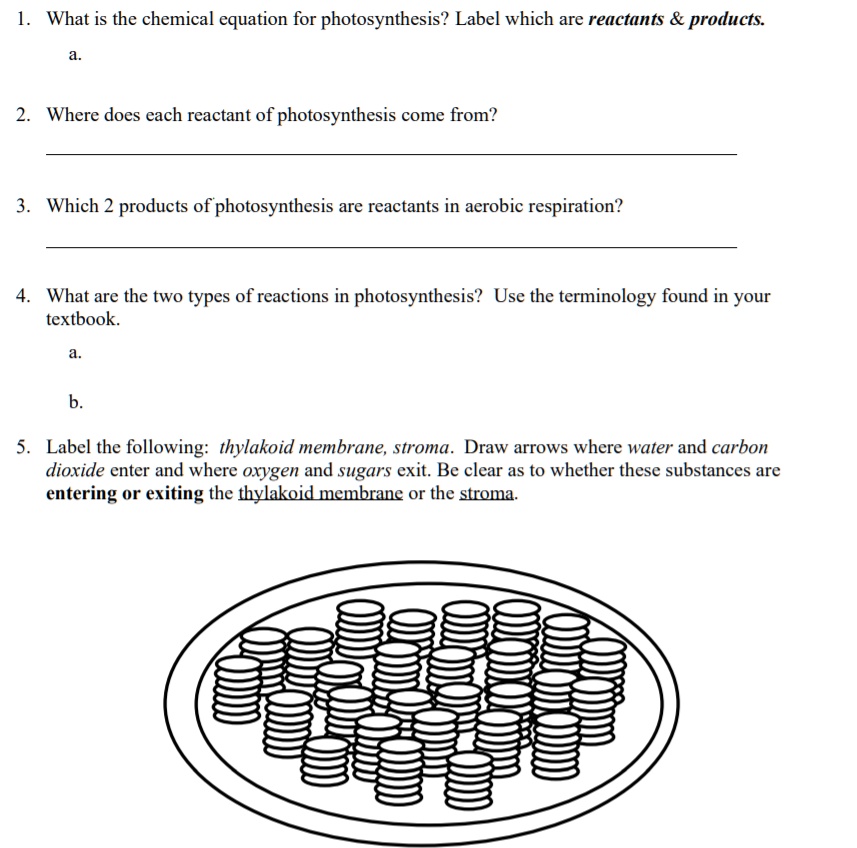
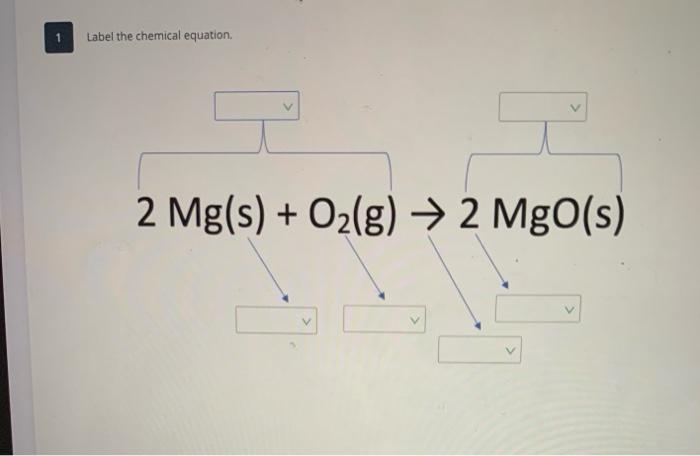
42 chemical equation with labels
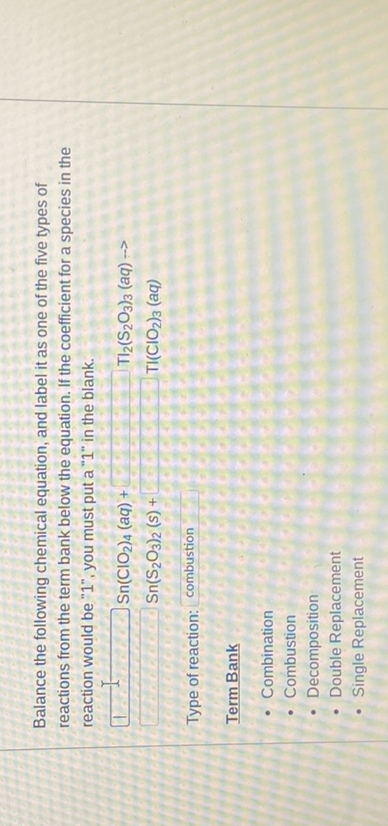
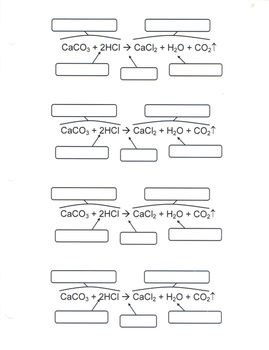
3.2.3: Everyday Life- Why Fats Don't Add Up on Food Nutrition Labels a. Calculate the mass of glycerol (G) produced when 3.84 mol GPPL is reacted with adequate H 2 O according to Equation (1). b. Show that a nutritional label for this fat would have trans, unsaturated, and polyunsaturated fats that don't add up to the total fat. Solution The problem asks that we calculate the mass of glycerol produced. Label the parts of the chemical equation with the appropriate ... Label the parts of the chemical equation with the appropriate descriptions. Sr(NO3)2(aq) + K2CO3(aq) ⟶ SrCO3(s) + 2KNO3(aq). answer bank-.
Chapter 4 Quantities of reactants and products Phase labels: letters written in parenthesis after a reactant or product to indicate whether the substance is a solid (s), liquid (l), gas (g) or dissolved in ...

Chemical equation with labels
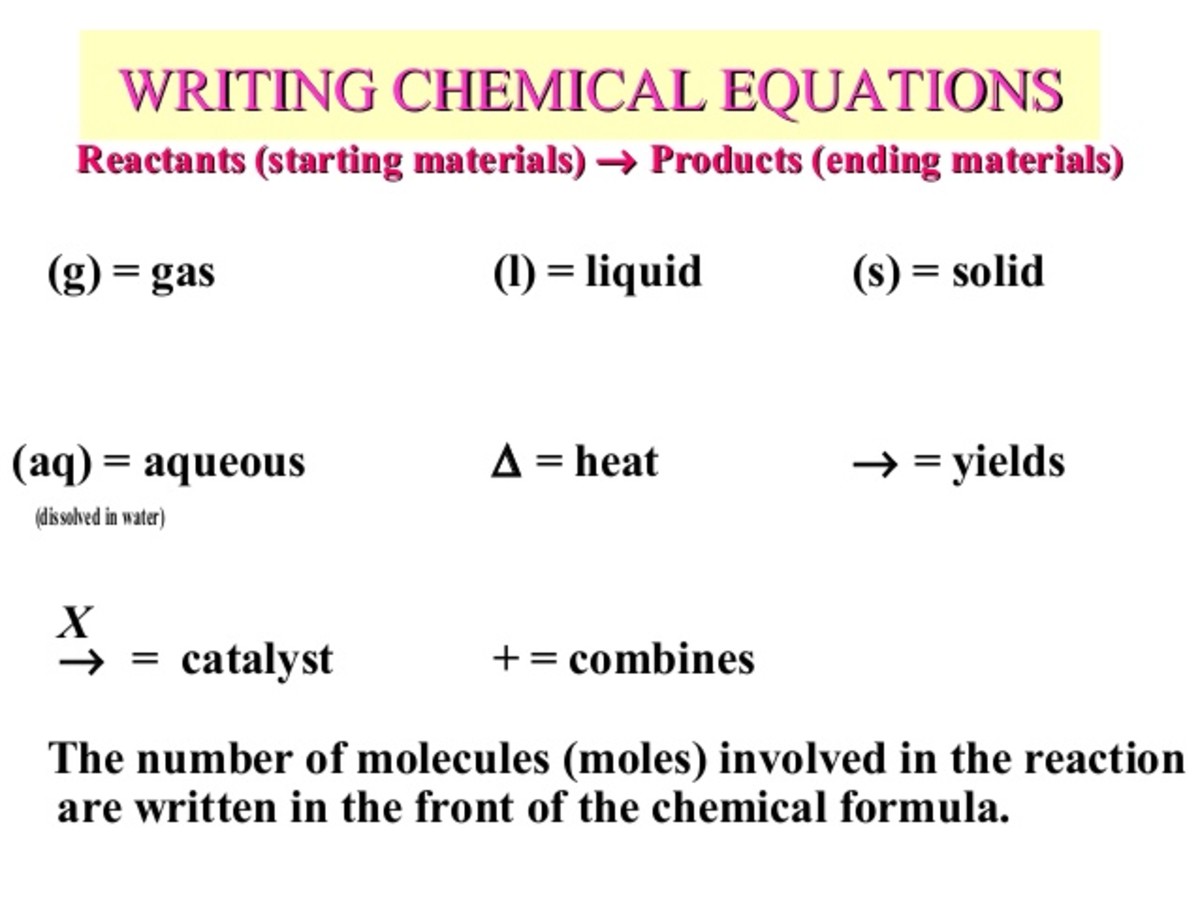
Definition of dissolve and the state label (aq) in chemical equations A chemical equation is not meant to convey everything. It is just a shorthand notation to describe chemical reactions. A real experiment or a research paper should describe the experimental condition with sufficient details in such a way that an experienced person should be able to repeat it. The Chemical Equation - GitHub Pages Test Yourself. Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer. N 2 + 3H 2 → 2NH 3. Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Chemical Equations - Let's Talk Science For a balanced chemical equation, the number of atoms on the left side of a chemical equation has to equal the number of atoms on the right side of the equation. We started off with H 2 (g) + O2 (g) on the right-hand side of the equation. This means that there are two atoms of hydrogen (H 2) and two atoms of oxygen (O 2 ): H2 (g) + O2 (g)
Chemical equation with labels. Balancing Chemical Equations Activity - Carolina Knowledge Center CH 4 + 2O 2 → CO 2 + 2H 2 O. 2K + 2H 2 O → 2KOH + H 2. HCl + NaOH → NaCl + H 2 O. FeS + 2HCl → H 2 S + FeCl 2. C 2 H 4 + 3O 2 → 2CO 2 + 2H 2 O. This straightforward activity should clear up any confusion that some students may still have between coefficients and subscripts in chemical equations. For additional student practice in ... How would you label each formula in the chemical equation below as ... Fe + S -> FeS | Socratic How would you label each formula in the chemical equation below as either a reactant or a product? F e + S → F eS Chemistry Chemical Reactions Chemical Reactions and Equations 1 Answer anor277 Jan 23, 2017 We designate the reactants as written........ Explanation: The reactants are on the LEFT HAND SIDE of the equation. How to Write a Chemical Equation (with Pictures) - wikiHow If you want to write a chemical equation, start by writing the chemical formulas of each reactant. Use the prefixes, such as mono-, di-, tri-, and tetra-, to figure out the number of atoms present for each element, and write this number as a subscript for each element. For example, dihydrogen monoxide would be more easily written as H2O. Diffusion MRI - Wikipedia Diffusion-weighted magnetic resonance imaging (DWI or DW-MRI) is the use of specific MRI sequences as well as software that generates images from the resulting data that uses the diffusion of water molecules to generate contrast in MR images. It allows the mapping of the diffusion process of molecules, mainly water, in biological tissues, in vivo and non-invasively.
How to write chemical formula with branching and with labels for each ... Here is a solution along the lines of what you were doing for giving equation style labels to tikz diagrams. It defines an option eqn-label for a node which puts an equation number on the right hand side of the page on level with the current node. This uses the current page node so you need to include the remember picture option in the tikzpicture options. IB Chemistry (Ellesmere College) - Past Paper Questions (ii) Draw an enthalpy level diagram for this reaction, including labels for Δ H Ө, E a and the activation energy when a catalyst is used, E cat. (4) (Total 12 marks) 26. Nitrogen (II) Oxide reacts with bromine according to the following equation. 2NO (g) + Br 2(g) → 2NOBr (g) DH = negative Chemical Equation Balancer The equation will now look like this: 4Na + O 2 = 2Na 2 O This equation is a balanced equation because there is an equal number of atoms of each element on the left and right hand sides of the equation. Example #2 (Complex) P 4 + O 2 = 2P 2 O 5 This equation is not balanced because there is an unequal amount of O's on both sides of the equation. Chemical equation - Wikipedia As seen from the equation CH 4 + 2 O 2 → CO 2 + 2 H 2O, a coefficient of 2 must be placed before the oxygen gas on the reactants side and before the water on the products side in order for, as per the law of conservation of mass, the quantity of each element does not change during the reaction P 4 O 10 + 6 H 2 O → 4 H 3 PO 4
Align and label in chemical equation - LaTeX Stack Exchange For the first objective, change from an align* to an align environment and use \notag directives on lines 1, 4, and 5. What are Chemical Equations? Detailed Explanation, Examples Chemical Equation: CaCl 2 + 2AgNO 3 → Ca(NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3 - → Ca 2+ + 2NO 3 - + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ (calcium ion) and the NO 3 - (nitrate) ions are present on both sides ... Harris Quantitative Chemical Analysis 8th edition - Academia.edu Harris Quantitative Chemical Analysis 8th edition. David Garcia. Download Download PDF. Full PDF Package Download Full PDF Package. This Paper. A short summary of this paper. 7 Full PDFs related to this paper. Download. PDF Pack. People also downloaded these PDFs. People also downloaded these free PDFs. 4.E: Chemical Reactions and Equations (Exercises) 4.1: The Chemical Equation From the statement "nitrogen and hydrogen react to produce ammonia," identify the reactants and the products. From the statement "sodium metal reacts with water to produce sodium hydroxide and hydrogen," identify the reactants and the products.
How to write a chemical formula? - TeX - Stack Exchange Aug 03, 2017 · I can attempt to write the chemical formula as a mathematical formula: \documentclass{report} \usepackage{amsmath} \begin{document} As the scintillator $\text{PbWO}_{\text{4}}$ crystals are used. \end{document} However, this method is a brute force when approaching a chemical formula. How would a versed Latex expert solve this task?
PDF Balancing Chemical Equations Using Models Label one plate reactants and the other products, to represent each side of a chemical equation. Use at least four different colors of skittles (representing atoms of different elements) to make models of the substances in each equation below. 4 Skittles . 2. Choose one color to represent an element in the following chemical reaction.
What is a Chemical Equation? - Definition & Examples To represent a generic chemical equation, we often use alphabetic letters. We read the equation as ''A plus B yields AB'' (or ''A plus B forms AB'' or ''A plus B goes to AB''). Note that A and B...
4.1 Writing and Balancing Chemical Equations - Chemistry The two dissolved ionic compounds, NaOH and Na 2 CO 3, can be represented as dissociated ions to yield the complete ionic equation: CO2(aq)+2Na+(aq)+2OH−(aq) 2Na+(aq)+CO32−(aq)+H2O(l) CO 2 ( a q) + 2 Na + ( a q) + 2 OH − ( a q) 2 Na + ( a q) + CO 3 2 − ( a q) + H 2 O ( l)
Welcome to CK-12 Foundation | CK-12 Foundation FlexBook Platform®, FlexBook®, FlexLet® and FlexCard™ are registered trademarks of CK-12 Foundation.
What Are Chemical Equations? - ThoughtCo This can be seen in the following equation: 2 H 2 (g) + O 2 (g) → 2 H 2 O (l) Hydrogen and oxygen are indicated by (g), which means they are gases. Water is marked (l), which means it is a liquid. Another symbol you may see is (aq), which means the chemical species is in water — or an aqueous solution. The (aq) symbol is a sort of shorthand ...
Chemical Equation | Reactants And Products In Chemical Reactions We will first take the maximum number of atoms present on either side of the reaction, that we can find on product side. (Oxygen = 4). Then we multiply the number of oxygen atoms on reactant side by 4, such that the number of oxygen atoms on both sides of the reaction is balanced. The equation becomes F e + 4 H 2 O → F e 3 O 4 + H 2
Quantitative Chemical Analysis 7E Daniel C. Harris Quantitative Chemical Analysis 7E Daniel C. Harris. Alfredo Morales. Download Download PDF. Full PDF Package Download Full PDF Package. This Paper. A short summary of this paper. 8 Full PDFs related to this paper. Read Paper. Download Download PDF.
pycse - Python3 Computations in Science and Engineering In the first call to the function, we only define the argument a, which is a mandatory, positional argument.In the second call, we define a and n, in the order they are defined in the function.Finally, in the third call, we define a as a positional argument, and n as a keyword argument.. If all of the arguments are optional, we can even call the function with no arguments.
Chemical Equations - GitHub Pages It is not uncommon to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note
Chemical formula - Wikipedia A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include ...
How do you label a chemical equations? - Answers What are chemical equations? chemical equations are a formal algebra that permits the calculation of chemical reactions.
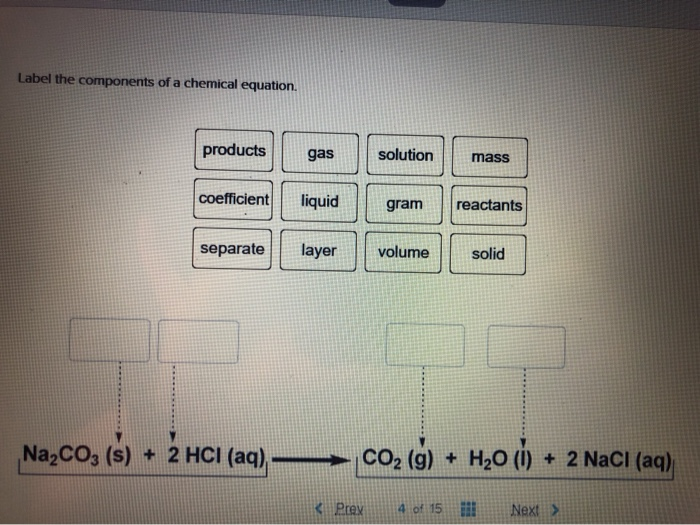
Solved Label the components of a chemical equation. separate | Chegg.com Chemistry Chemistry questions and answers Label the components of a chemical equation. separate mass liquid coefficient gas solution solid reactants layer prod uiets volume gram Na2co, (s) + 2HCl (aq),- ?.co2 (g) + H2O (l) Co2 (g) + H20 ) + 2 Naci (aq)
Label the chemical Equation worksheet - Liveworksheets.com Label the chemical Equation Label the parts of the chemical Equation ID: 1570824 Language: English School subject: Science Grade/level: 5 Age: 7-9 Main content: Chemical Equation Other contents: NA Add to my workbooks (0) Download file pdf Embed in my website or blog
How to Number or Label Equations in Microsoft Word In the New Label window, type your left parenthesis and hit "OK." If you want to select a different number format, click "Numbering," choose what you'd like to use, and click "OK." You'll see the starting parenthesis with the first number per the formatting that you selected. Type a space, and then your closing parenthesis.
What are the Parts of a Chemical Equation? - Life Persona 2 - Begin by writing the chemical equation in terms of the substances involved: C 2 H 6 T the 2 → CO 2 + H 2 OR We have two carbon atoms left, so we need two molecules of carbon dioxide on the side of the product, so that each side has two carbon atoms. That element is balanced.
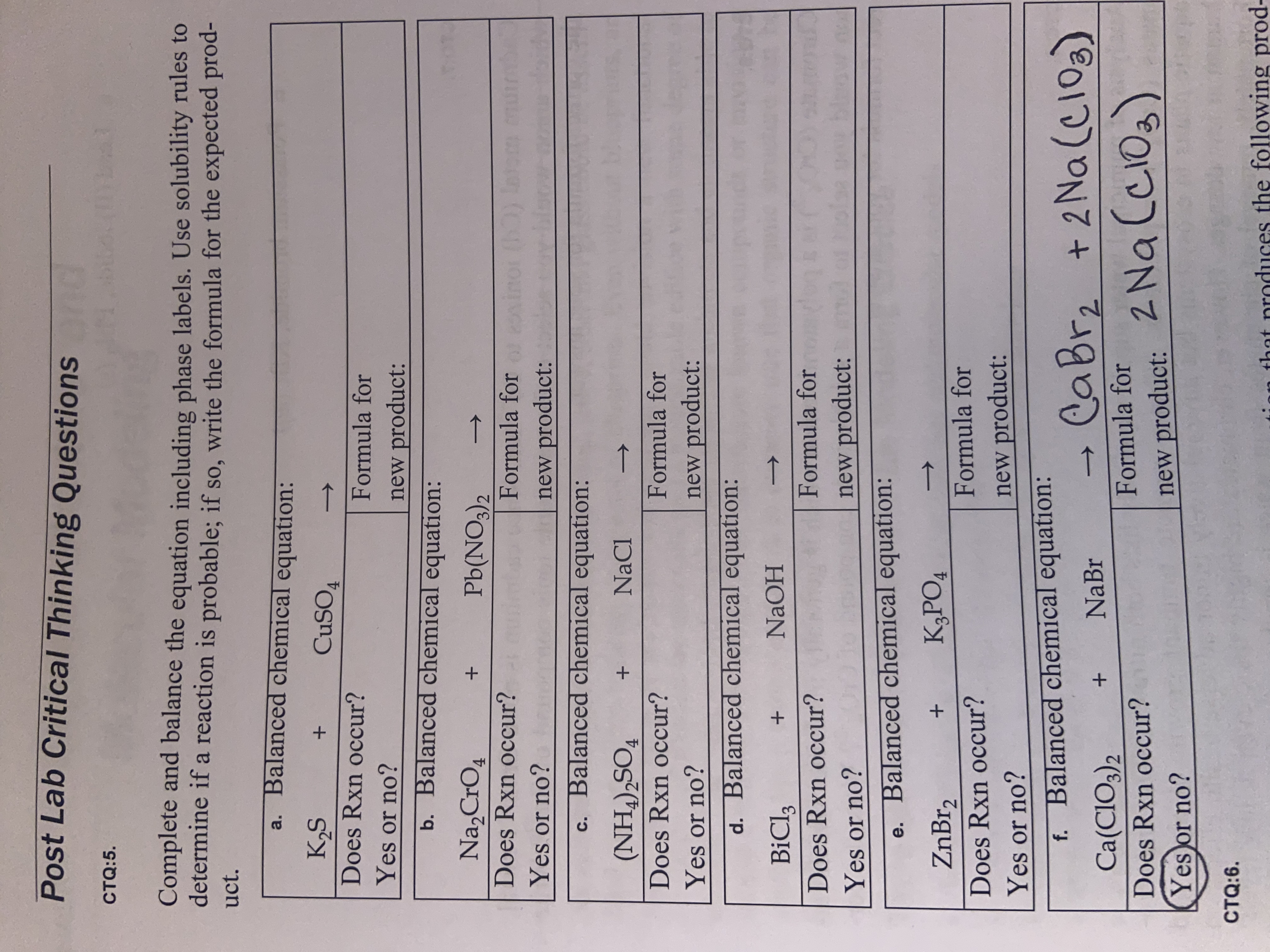
Solved For the chemical equations shown below, label each | Chegg.com Drag each label to the appropriate target. HPO_4^2- (aq) + H_2O (l) H_2PO_4^- (aq) +OH^- (aq) HPO_4^2- (aq) +H_2O (l) PO_4^-3 (aq) + H_3O^+ (aq) Question: For the chemical equations shown below, label each reactant as either acid or base, and each product as either conjugate acid or conjugate base according to the Bronsted-Lowry definition.
How to Balance Chemical Equations - Study.com The equation is not balanced because in the reactants side, there are 2 nitrogen (N) atoms and 2 hydrogen (H) atoms. In the products side, there are 1 nitrogen (N) atoms and 3 hydrogen (H) atoms.
3.1: Chemical Equations - Chemistry LibreTexts 15 Jun 2022 — A chemical reaction is described by a chemical equation, an expression that gives the identities and quantities of the substances involved in a ...
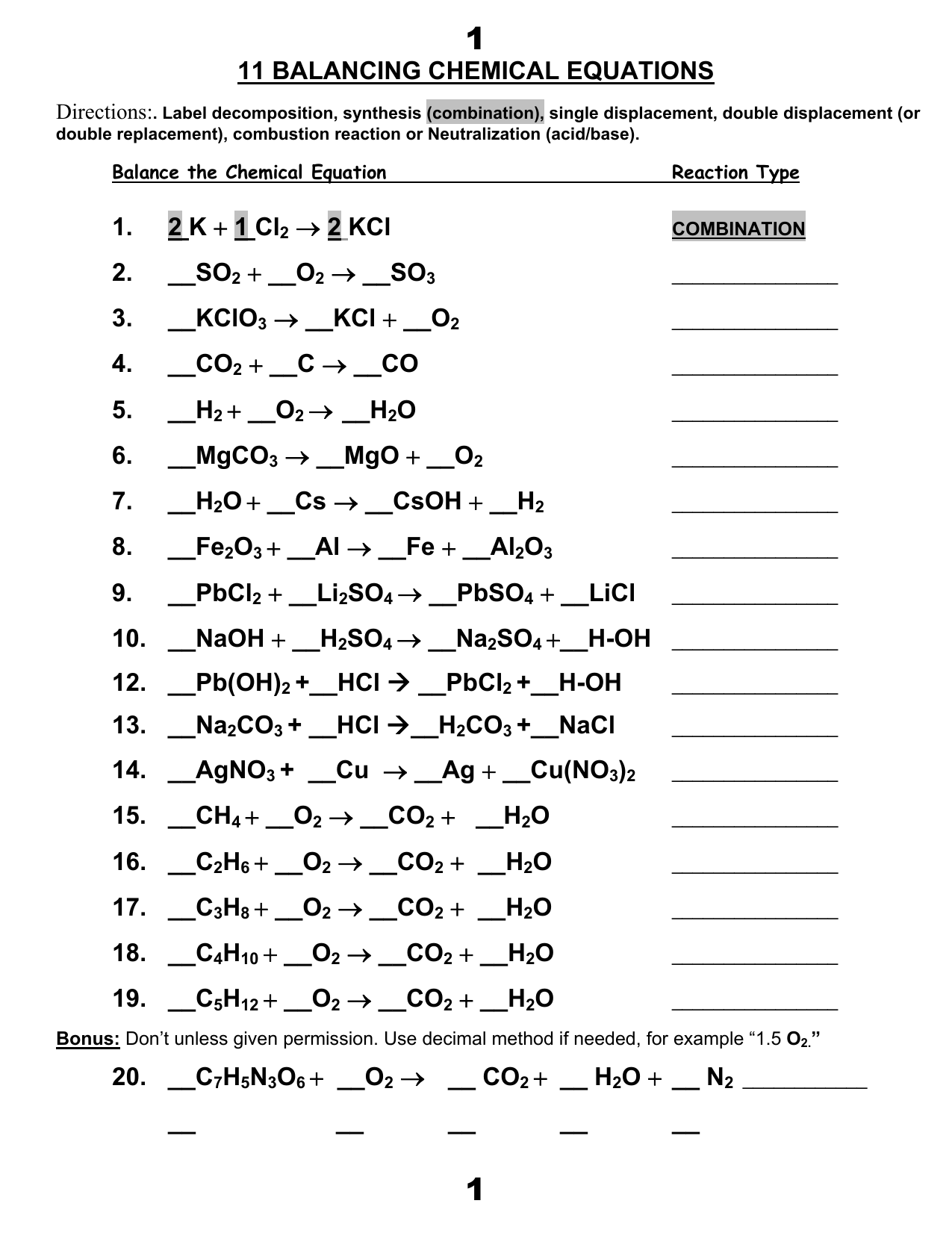
Examples of Balanced Chemical Equations - ThoughtCo Writing balanced chemical equations is essential for chemistry class.Here are examples of balanced equations you can review or use for homework. Note that if you have "1" of something, it does not get a coefficient or subscript. The word equations for a few of these reactions have been provided, though most likely you'll be asked to provide only the standard chemical equations.
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Start by writing the chemical equation in terms of the substances involved: C 2 H 6 + O 2 → CO 2 + H 2 O We have two carbon atoms on the left, so we need two carbon dioxide molecules on the product side, so that each side has two carbon atoms; that element is balanced.
The Canadian Journal of Chemical Engineering Chemical compounds are to be named according to the rules established by IUPAC. Positional prefixes are to be printed in italics. Example: “n-butane, n-C 4-H 10.” Equations. All equations should be numbered using Arabic numerals and correctly punctuated. Refer to equations as “Equation (1)” (not “Eq. (1)” or “Equation 1”).
How do you Write a Chemical Equation? - A Plus Topper The products of reaction are written in terms of their symbols or molecular formulae on the right-hand side of the equation. A plus (+) sign is added between the formulae of the products. In between the reactants and the products an arrow sign ( ) is inserted to show which way the reaction is occurring. A + B C + D
Chemical Equations - Let's Talk Science For a balanced chemical equation, the number of atoms on the left side of a chemical equation has to equal the number of atoms on the right side of the equation. We started off with H 2 (g) + O2 (g) on the right-hand side of the equation. This means that there are two atoms of hydrogen (H 2) and two atoms of oxygen (O 2 ): H2 (g) + O2 (g)
The Chemical Equation - GitHub Pages Test Yourself. Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer. N 2 + 3H 2 → 2NH 3. Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water).
Definition of dissolve and the state label (aq) in chemical equations A chemical equation is not meant to convey everything. It is just a shorthand notation to describe chemical reactions. A real experiment or a research paper should describe the experimental condition with sufficient details in such a way that an experienced person should be able to repeat it.














![[MK]EMILEE Fashion Chemical Equation Brooch Art Denim Jackets La di Nadine Ross | Tokopedia](https://images.tokopedia.net/img/cache/500-square/hDjmkQ/2022/3/19/8615f66a-76d0-417b-942f-fe90c91e4e8b.jpg)






Post a Comment for "42 chemical equation with labels"